CE MARKING | UKCA Mark
CE MARKING | UKCA Mark

Understanding CE Marking for Industrial Products in Europe
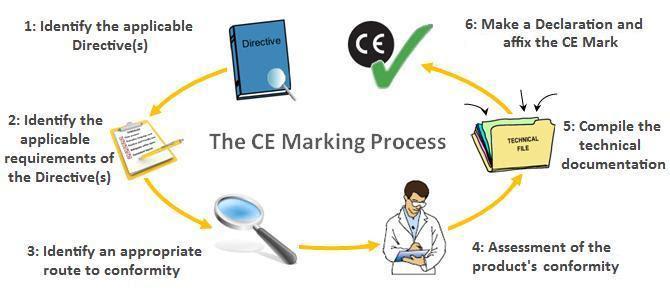
In the vast landscape of industry and commerce, ensuring the safety and compliance of your products is of paramount importance, especially if you plan to introduce them to the European Economic Area (EEA). The CE Mark, short for "Conformité Européenne," serves as a prominent symbol of compliance with EU Product Safety Directives. In this comprehensive guide, we will delve into the world of CE Marking, understanding what it entails, and how CE Industrial Services can be your guiding light in navigating the complex terrain of conformity.
Unraveling the CE Mark: What Does It Signify?
The CE Mark is not just a symbol; it's a declaration. It signifies that the product to which it is affixed complies with the stringent regulations set forth by the European Union. These regulations, often referred to as EU Directives, apply to all products that are being introduced into the EEA for the first time. To make this concept clearer, let's explore the CE Mark in detail.
EU Directives: The Backbone of CE Marking
EU Directives are a set of guidelines and standards that govern the safety and performance of products within the EEA. Understanding which directives apply to your product is the first step towards achieving CE Marking. Let's take a closer look at some of the critical directives that may be relevant to your industrial products:
1. Medical Device Directive
This directive outlines the requirements for medical devices, ensuring their safety and efficacy.
2. In-Vitro Diagnostic Device Directive
It pertains to diagnostic devices used for in-vitro examinations, ensuring their reliability and accuracy.
3. Active Implantable Medical Device Directive
This directive concerns products that are implanted into the human body, imposing stringent safety measures.
4. Machinery Directive
It focuses on the safety of machinery and equipment used in various industries.
5. Pressure Equipment Directive
This directive deals with the safe design and manufacturing of pressure equipment.
6. Low Voltage Directive
It ensures that electrical equipment operates safely at low voltage levels.
7. EMC Directive
This directive addresses electromagnetic compatibility, crucial for electronic products.
8. Transportable Pressure Equipment Directive
It applies to pressure equipment designed for transport, guaranteeing their safety during transit.
9. Lift Directive
This directive covers the safety of lifts and elevators, a vital aspect in buildings and industries.
10. Construction Products Directive
It regulates construction products to ensure they meet safety and performance standards.
11. Toys Safety Directive
Toys must adhere to this directive to ensure the safety of children during play.
12. Personal Protective Equipment Directive
It outlines safety requirements for personal protective equipment, such as helmets and gloves.
The EC Declaration of Conformity: A Crucial Document
To obtain the CE Mark, a manufacturer must provide an EC Declaration of Conformity. This document is a written statement demonstrating that the product complies with all relevant EU requirements. It should contain detailed information for the identification of the related Community harmonization legislation. Manufacturers or their European Authorized Representatives (if based outside the EU) must retain this declaration.
Navigating the Complexities
CE Marking is not a straightforward process. Many complexities can arise, including determining which EU Directives apply, understanding the need for a Notified Body, assessing product safety, and compiling necessary manuals. Safeguarding industrial machines and products is a multifaceted endeavor, and this is where CE Industrial Services comes into play.
CE Industrial Services: Your Compliance Partner
CE Industrial Services is your dedicated partner in achieving CE Marking for your industrial products. We specialize in CE mark class:1 certification and, while we are not a Testing Lab nor a notified body, our expertise lies in guiding you through the maze of regulations and requirements.
Our experienced team understands the intricacies of CE Marking and can assist you in identifying the applicable directives, preparing the EC Declaration of Conformity, and ensuring that your products meet the EU standards. With CE Industrial Services by your side, you can navigate the CE Marking process with confidence.
Conclusion
In the realm of industrial products, achieving CE Marking is not just a requirement; it's a testament to your commitment to safety and compliance. CE Marking signifies that your products adhere to the stringent EU Directives, ensuring their safety and reliability. By partnering with CE Industrial Services, you can streamline the CE Marking process and confidently introduce your products to the European Economic Area.
FAQs (Frequently Asked Questions)
1. **What is the significance of the CE Mark?**
The CE Mark indicates that a product complies with EU Product Safety Directives and is safe for use in the European Economic Area.
2. **How do I determine which EU Directives apply to my product?**
Identifying relevant directives can be complex. Consulting with experts like CE Industrial Services can help you navigate this process.
3. **Is a Notified Body required for CE Marking?**
In some cases, yes. The need for a Notified Body depends on the product type and associated risks.
4. **What does the EC Declaration of Conformity entail?**
The EC Declaration of Conformity is a written statement by the manufacturer, confirming compliance with EU requirements. It must contain detailed information on related legislation.
5. **What services does CE Industrial Services provide?**
CE Industrial Services specializes in CE mark class:1 certification and offers expert guidance on CE Marking for industrial products in the EEA. While not a Testing Lab nor a notified body, they assist in compliance efforts.